Day 2 :
Keynote Forum
Anita Yadav
University of Mumbai, India
Keynote: Contemporary drug discovery and design
Time : 09:15-10:00

Biography:
Abstract:
Keynote Forum
Mohammed Salama Al-Ajely
Mosul University, Iraq
Keynote: Polymer and heterocyclic compounds their utility and applications as drug
Time : 10:00-10:45

Biography:
Abstract:
- Drug Discovery Using Nanotechnology | Pharmaceutical Research & Development ( R&D ) | Anti-Cancer Drug Discovery | Natural Products Drug Discovery | Drug Design and Drug Development

Chair
Heyam S.Ali
Dubai Pharmacy College, Dubai
Session Introduction
Mustafa Guzel
Istanbul Medipol University, Turkey
Title: Novel targets for cancer metabolism
Time : 11.30-12.05

Biography:
Mustafa Guzal has 14 years of teaching experience, 7 years at college level chemistry/organic chemistry/medicinal chemistry courses and related laboratory courses (Clemson University, Northeastern University, and currently Istanbul Medipol University), and 6 years of chemistry teaching experience at various high schools complemented with 14 years of industrial research experience in medicinal chemistry with increased responsibilities and various positions at pharmaceutical companies. (ArQule and TransTech Pharma Inc.currently vTv Therapeutics
Abstract:
Cancer remains the second leading cause of death in the world after heart disease and cardiovascular complications. Moreover, survivors of cancer continue to suffer from symptoms of pain, fatigue and depression despite existing treatment advances for cancer treatment. Even though numerous pharmacological therapies have been developed in the past decade, the advantage of new treatment options remains important in the fight against this deadly disease. It is now well understood that protein kinases play key roles in the growth and survival of cancer cells by regulating their onset of DNA synthesis, their response to DNA damage and their entry, progression and exit from mitosis. Clinical validations prove that protein kinases are an attractive class of therapeutic drug targets for cancer as demonstrated with the recent approval of six protein kinase inhibitors. The Warburg effect describes the reliance of cancer cells on glycolysis for energy. Increased glycolysis and acid resistance have been postulated to be an essential part of carcinogenesis, conferring a significant growth advantage as well as promoting typical tumor progression. Targeting accelerated glycolysis in cancer cells is a new promising modality for treatment of cancer. Inhibition of glycolysis can be done without significant side effects and such treatment will be additive to most known cancer therapies. Recent studies show that methyl jasmonate reveals promising results for treatment of cancer. During the presentation, the role of aerobic glycolysis for tumor growth and small molecule drug discovery and development efforts as well as their therapeutic applications for oncological indications will be highlighted. The structure of this targeted compounds shall not yet be disclosed due to nature of intellectually property issues, however during the presentation some of those lead compounds will be revealed.
References
- Coller A H (2014) Is cancer a metabolic disease. The American Journal of Pathology; 184(1): 4-17.
- Lu W, Logsdon C D and Abbruzzese J L (2013) Cancer metabolism and its therapeutic implications. Journal of Cell Science & Therapy; 2(2): 1-10.
- Heiden M G V (2011) Targeting cancer metabolism: A therapeutic window opens. Nature Reviews: Drug Discovery; 10: 671-684.
- Cohen S and Flescher E (2009) Methyl jasmonate: A plant stress hormone as an anti-cancer drug. Phytochemistry; 70: 1600-1609.
- Pathania D, Millard M and Neamati N (2009) Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Advanced Drug Delivery Reviews; 61: 1250-1275.
Manuela Stoicescu
University of Oradea, Romania
Title: The response to the administration of insulin is personalized - genetic determined
Time : 13:30-13:55

Biography:
Manuela Stoicescu is a Consultant Internal Medicine Physician. She has received her PhD in Internal Medicine and currently, she is an Assistant Professor of University of Oradea, Faculty of Medicine and Pharmacy, Medical Disciplines Department, Romania. She was invited as a speaker at more than 30 International Conferences is USA, China, Japan, Canada, Thailand, Dubai, Spain, Germany, etc. She is a committing organizing member at many International Conferences and an editorial board member in two ISSN prestigious Journal in USA. She has published more than 20 articles in prestigious ISSN Journals in USA, has published five books: two books for students, two books on Amazon at International Editor-LAP Lambert Publishing Academic House in Germany, one monograph entitled ‘High blood pressure in the young a ignored problem’ and two book chapters.
Abstract:
One of the most difficult problems in the medical practice is to establish the necessary of the insulin at a patient who need insulin therapy, indifferent that this is a young patient with type 1 insulin-depended diabetes mellitus or a patient with type 2 diabetes mellitus who in one moment need insulin administration – insulin necessitate. Theoretically appear simple in the first instance, because we have a standard protocol to calculate the necessary of insulin like 0,3 UI insulin/kg body and after that to devised the doses depend what type of scheme we want to start: an intensification scheme in four or three administrations per days fast insulin and one Lantus insulin at 21 a clock - more physiologically or an conventional scheme in two prizes of NPH insulin in the morning and afternoon and the doses depend the body mass of a person. But, practical experience showed that after scheme of insulin was started, the patient must to be very carefully monitories and supervise to adjust the doses of insulin in compliance with the glycemic profile of the patient, because hypoglycemia can appear unexpected any time especially after the intensification scheme and is very dangerous. For this reason the doctors all the time must to adjusted the dosages of insulin follow the glycemia profile if appear dawn phenomenon or Sömögyi phenomenon and to change the insulin dosages to avoid dangerous hypoglycemia. But also in the end of the scheme when insulin necessary is established, the patient isn’t yet in safe condition because a hypoglycemic accident can appear anytime. Of course we know that any other many factors can determine this like: physical efforts, stress, lunch (carbohydrate intake) and others but in this moment we must to understand that the response to insulin administration it is personalized and genetic determined.
Results and discussions: In this moment the decrease level of glycemia after insulin therapy is unpredictable. We can’t appreciate exactly how much follow to decrease the level of glycemia after was administrated the same quantity (units of insulin) at the same patient with the same body mass and the same level of glycemia. The response is different, but we expect to be the same. For this reason exist a specific response of every person, personalized and genetic determined. I believe with strong opinion that only in moment when we will know the genome of the patient (the genetic profile), to can calculate the genetic necessary of insulin of every person we can establish the truth and will be avoid so many dangerous hypoglycemic accidents in present.
Anita Yadav
Mumbai University, India
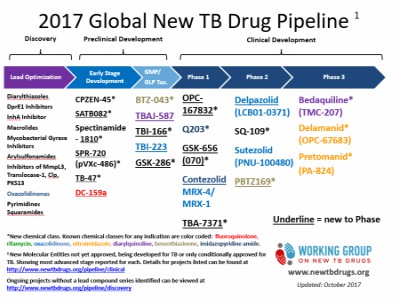
Title: Development Of Novel Antitubercular Drugs: Current Status
Time : 14:20-14:45

Biography:
Anita Yadav has her expertise in computer aided drug designing. She has worked on Structure Based Drug Designing, Ligand Based Drug Designing, 2D, 3D and Group QSAR based Drug Designing, De-Novo Designing, Homology Modelling, Pharmacophore modelling, Lead Identification and QSPR
Designing. She can design any agonist and antagonist for the resistant bacterial strains. She has worked on Synthesis, Purification, and Structural Elucidation & Pharmacological Evaluation of following classes of compounds: Anti-tubercular, Anti-diabetic, Anti-malarial, Anti -inflammatory and Antibacterial agents.She is Working as “Assistant Professor in Pharmaceutical Chemistry” at Dr. L. H. Hiranandani College of Pharmacy, Ulhasnagar (Affiliated to Mumbai University). She won many prizes for her research.She has 1 international and 3 national publications also 2 international and 21 national presentations to her credit.
Abstract:
Tuberculosis (TB) is one of the most ancient diseases of mankind and has co-evolved with humans for many thousands of years. Drug-resistant TB is a continuing threat. Modern chemotherapy has significantly improved patient outcomes against drug sensitive tuberculosis. However, the rapid emergences of drug-resistant tuberculosis, together with the bacterium’s ability to persist and remain latent present a major public health challenge. To overcome this problem, research into novel anti-tuberculosis targets and drug candidates is thus of paramount importance. Recent advances in molecular tools make possible the identification of targets essential for survival and persistence whose inhibition is likely to shorten therapy. Various new drug targets and drug candidates have been recently reviewed. Currently there are a number of drug candidates in different phases of the discovery, pre-clinical and clinical development. There are also a number of ongoing trials using repurposed drugs, where different combinations and doses of drugs that are currently on the market, are being tested with a view of optimizing therapies.
Recent Publications
-
Chaudhari R Y, Bhise S B, Yadav A S, Sonawane T (2016) QSAR, Synthesis and Docking Study of 1,4-DHP as Novel Antitubercular Agents JPRCP (6).
-
GHODKE YOGITA DEEPAK* & YADAV ANITA SHASHIKANT (2014) Extraction And Pharmacological Screening Of Carvone And It’s Derivatives, IJPA- 3(1).
-
YADAV ANITA * , MAGDUM C. S. (2014) SYNTHESIS AND BIOLOGICAL ACTIVITY OF 1, 6-DIHYDROPYRIDINE DERIVATIVES. IJPA (3).
-
Yadav Anita Shashikant and Ghodke Yogita Deepak (2013) KAJAL: COSMECEUTICAL, IJPA 2(9).
Meshari Alrayees
King Salman Armed Forces Hospital-North Western Region, KSA
Title: The importance of implementing clinical design support system while using electronic prescriptions
Time : 15:10-15:35

Biography:
Meshari Alrayees has his expertise in pharmaceutical care in improving the patient safety and quality of service in healthcare system. He has built his knowledge after years of experience in research and administration work in tertiary hospital and education institutions. He has his Bachelor of Pharmaceutical Science from King Saud University in KSA as well as graduated JCI Diploma and Master of Pharmaceutical Science from University of Tasmania in Australia. He is the Director of Continuous Quality Improvement and Patient Safety in King Salman Armed Forces Hospital in North Western Region in KSA and a former Head of Department of Pharmacy in this tertiary hospital. He is interested in the field of improving the patient safety and quality of service provided.
Abstract:
Statement of the Problem: Interventions by the pharmacists in prescribing process have always been considered as a valuable input by the health care community in the patient care process by reducing the medication errors and rationalizing the therapy. Researchers have reported that introduction of electronic prescription system clearly reduces medication prescription errors. However, the clinical benefits of clinical design support system (CDSS) with electronic prescriptions have been previously studied and proofed to improve the quality of prescribing.
Purpose: The purpose of this study is to describe the experience of using electronic prescriptions without CDSS and to show the importance of pharmacist intervention and CDSS as added prevention layers for medication errors.
Methodology & Theoretical Orientation: It is retrospective chart review about the pharmacist’s interventions over the period of 1 year. All the data is generated in the computer. Simple random sampling technique was used and the sample size was 592 prescriptions. Data gathered electronically and analyzed using SPSS version 21 to categorize the medication prescribing errors and related factors.
Findings: The benefit of using electronic prescribing system was limited to enhance the prescribing process but not improving the quality of prescribing pattern entirely. The pharmacist plays an important role as additional prevention layer for any prescribing error by catching medication errors mainly because of lack of knowledge of the prescribers.
Conclusion & Significance: Electronic prescribing system is a worthy system to be used to reduce the medication prescribing errors. However, CDSS should be implemented in this system because it is an integral part to ensure patient safety and improve the prescribing service.
Recent Publications
1.Mohamed H Ragab, Mohammed Y Al-Hindi and Meshari M Alrayees (2016) Neonatal parenteral nutrition: Review of the pharmacist role as a prescriber. Saudi Pharm J.; 24(4): 429-440.
2.Alatawi Y M, Kavookjian J, Ekong G and Alrayees M M (2016) The association between health beliefs and medication adherence among patients with type-2 diabetes. Res Social Adm Pharm.; 12(6): 914-925.
Hytham M Ahmed
Menoufia University, Egypt
Title: CZE and HPTLC methods for simultaneous determination of Candesartan in binary mixtures with Amlodipine and Hydrochlorothiazide
Time : 13:55-14:20
Biography:
Hytham M Ahmed is a professor in pharmaceutical analysis department at Minoufiya University, He publishes more than 25 articles and and 3 projects under analysis department
Abstract:
Two methods were developed for the simultaneous determination of the antihypertensive drugs Candesartan (CAN), Amlodipine (AML) and Hydrochlorothiazide (HCT) in their combined tablet dosage form. Method-1: Capillary zone electrophoresis (CZE) was performed. Electrophoretic conditions were optimized to improve separation, sensitivity and rapidity. The proposed method used a fused silica capillary (70 cm × 75 μm id) and the background electrolyte was 40 mM phosphate buffer pH 8, with 9 s injection time. The applied voltage was 30 kV. The diode array detector (DAD) was set at 210 nm for measurement of AML and CAN and 225 nm for HCT. The three compounds were resolved in less than 7 min with migration times 4.18, 5.36 and 6.74 min for AML, HCT and CAN, respectively. The described method was linear over the range of 5-100, 2.5-100 and 2.5-50 μg/mL for AML, HCT and CAN, respectively with correlation coefficients >0.9993. Method-2: HPTLC analysis was carried out on aluminum-backed sheet of silica gel using chloroform, methanol and ammonia in the ratio (8:2:0.2) as mobile phase. Retardation factors were 0.35, 0.5 and 0.75 for HCT, CAN and AML, respectively. Quantification was achieved with UV densitometry at 365, 265 and 274 nm for AML, CAN and HCT, respectively. The linearity ranges were 0.1-0.7, 0.05-0.5 and 0.025-0.5 µg/spot for AML, CAN and HCT, respectively with correlation coefficients >0.9992. The analytical performance of both methods was thoroughly validated according to International Conference on Harmonization (ICH) guidelines with respect to system suitability, linearity, ranges, precision, accuracy, specificity, robustness, detection and quantification limits. The proposed CZE and HPTLC methods were successfully applied to estimation of the drugs in laboratory prepared mixtures and in their combined tablets dosage form. No chromatographic interference was observed from the tablets excipients.
Mohammad salman Al-Ajely
Mosul University, Iraq
Title: Synthesis of some heterocyclic compounds derived from furfural
Time : 12:05-12:30

Biography:
Mohammad salman Al-Ajely of University of Mosul, Mosul with expertise in Dental Hygiene and Epidemiology, Green Chemistry, Chemical Biology, mosul, Iraq. I also have many publications in polymer synthesis and applications I have 4 patent post doc in Australia Lat Robe University. Education.
Abstract:
The present investigation includes the synthesis of number of 5,6 membered ring heterocyclic compounds containing one , two hetero atom such as pyrrole, thiazole , oxadiazole , thiadiazole. It include the Synthesis of 2-Methyl Sulfonyl-5-bromo-6-(2-phenyl amino)-1,3,4oxadiazole-5-yl-1,3-pyrimidine (E9) from the reaction of 5-methylisothiouriaSulfate(E2) with MBA to form the corresponding carboxylic acid (E3).This acid was then transformed into the corresponding hydrazide (E9) after a series of reaction processes. The resulting hydrazide was then reacted with isothiocyanate to yield the corresponding thiosemicarbazide (E8) .Cyclization of this compound using mercuric oxide lead to the formation of oxadiazole derivative (E9) , Synthesis of 2- Methyl sulfonyl -4- (N- formyl carbohydrazido) -5- bromo pyrimidine (E10) through the reaction of compound (E7) with formic acid, then cyclization of the product (E10) using phosphorous peta sulfide into thiadiazole (E11). This pathway was also includes the synthesis of carboxim –ido substituted pyrimidines (E15-E17) from the reaction of dimidone with (E12-E14) ,The resulting product was then allowed to react with compound (E4)to yield (E15-E17) compounds. The other part of this pathway include the synthesis of amidoxime (E18- E20) which then allowed to react with (E3) using DCC to form 2- methylthio -5-bromo-6-substituted amino oxmyl-1,3-pyrimidine-4- carboxylates( E21- E23) these carboxylates were cyclized by DMSO into 2- methyl thio -5- bromo-6-(3-substituted-1,2,4-oxadiazole-5-yl)-1,3-pyrimidine (E24-E26) , The work also include the synthesis of 3,4- dibromo crotono lactone -5-( substituted) carboxylate(E27-E29) from the reaction of MBA with either acetyl chloride , benzoyl chloride or nitro benzoyl chloride ,The compound (27) was then allowed to react with secondary aliphatic amines to form 5-(substituted amino)-3,4-dibromo crotono lactones(E30-E32). This pathway also includes the reaction of compound(28)with substituted anilines to give 4- (substituted anilino)-3-bromo-5-benzoyloxy crotono lactones(E33-E36).
Ihmood KH. Jebur
Tikrit University, Iraq
Title: Synthesis and characterization of some new ethyl-2-amino benzothiazole -6-carboxylate derivatives
Time : 14:45:15-10

Biography:
Ihmood K H Jebur is Assistant Professor in Organic Chemistry at Tikrit University. He focuses in his area of research on heterocyclic chemistry. He has published many papers in this field. Currently he is teaching many graduate and undergraduate classes.
Abstract:
In this research we chosen a number of heterocyclic compounds 4-amino benzoate (procaine) (A1), the ester was synthesized by esterification of 4-amino benzoic acid with ethanol, this compound was then treated with potassium thiocyanate followed by oxidative cyclization of the resulted thiourea with bromine solution to afforded ethyl-2-aminobenzothiazole-6-carboxylate (A2). This compound was further treated with some substituted benzaldehyde yielding the Schiff bases (A3 a-d). Compound (A2) was also treated with acetic anhydride giving the corresponding N-(ethyl-2-amino benzothiazolyl-6-carboxylate acetamide (A4). Compound (A2) was allowed to react with hydrazine hydrate afforded 2-benzothiazolyl-6- hydrazido acetamide (A5), which was then condensed with substituted aromatic aldehydes affording 2-acetamido-6-benzothiazolyl-carbonylaryl hydrazine as a final product(A6 a-d). All the synthesized compounds were characterized by IR, some selected samples were checked by their elemental analysis, (CHN) and some of them by 1H-NMR method they also well discussed.
Heyam Saad Ali,
Dubai Pharmacy College Dubai
Title: Emerging applications of nanotechnology for diagnosis and therapy of disease
Time : 16:00- 17:00

Biography:
Dr. heyam Saad Ali, M. Pharm., and Ph-D from U of K and Bradford universities, UK. She is working as a head of pharmaceutics department in Dubai Pharmacy College, UAE. Prof. contributed more than 50 articles to reputed international scientific journals in different conventional, controlled and targeted drug delivery systems in pharmaceutical product development. She has been invited as speaker to numerous International conferences .Reviewer and member of editorial board of many international journals.
Abstract:
Nanotechnology is being used in the pharmaceutical field for different reasons. The main reason is to improve drug solubility and delivery to various sites for action. Nanotechnology is also used to develop new and improved therapeutic devices. Therapeutics are improved through both the consistent drug delivery, drug targeting and bioavailability of existing medicinal moieties, including the discovery of completely new substances and nanomaterials, which brings out different added advantages over classical drug delivery systems, including the targeted delivery to effected sites only, and the improved adsorption, distribution and duration of medication in the body 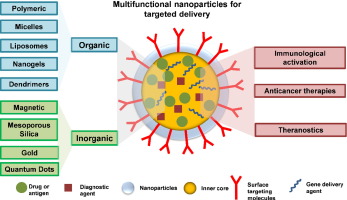
- 1-Nanomedicine
- 2-Nano particles in drug delivery
- 3-Pharmaceutical nanotechnology
- 4-Nanotechnology drug delivery systems
- 5-Nanotechnology and therapeutic delivery.
- 6-Applications of nanotechnology.
- Hot Topics in Drug Targets | Green Techniques for Medicinal Chemistry | Advance Trends in Organic Chemistry & Inorganic Chemistry | Traditional Chinese Medicine Discovery
Session Introduction
Shaikha S AlNeyadia
1UAE University Al-Ain, UAE
Title: Novel pyrimidine derivatives of rhodanine as pparγ agonists: Design, synthesis, molecular docking and glucose uptake
Time : 11:55-12:20

Biography:
Shaikha S Al Neyadi has obtained her PhD degree in 2016 from United Arab Emirates University. Her current research interest focus is on design and synthesis of bioactivity of novel derivatives as anti-diabetic and anti-bacterial drugs.
Abstract:
Rhodanines has become a very interesting class of heterocyclic compounds since the introduction of various glitazones and epalrestat into clinical use for the treatment of type II diabetes mellitus and diabetic complications, respectively. Chemical modifications of these hetero-cycles constantly result in compounds with a wide spectrum of pharmacological activities. In this study, a novel series of pyrimidine derivatives of rhodanine were designed, synthesized, docked against the PPARγ receptor target and their anti-diabetic activities evaluated. It was observed that three compounds 4e, 4f and 4g showed significantly good in vitro anti-diabetic activity in comparison to pioglitazone as reference drugs. Compound 4g was found to be the most active candidate in lowering blood glucose level and exhibited higher glucose uptake than the reference drug Pioglitazone. The structure-activity relationship and molecular docking analysis revealed that the carboxylate group could interact with Arg 288 and was favorable for interaction with the residues in PPARγ binding site and Compound 4g obtained the best docking score which was equal to -9.6 kcal/mol and able to score even better than the reference PPARγ agonist pioglitazone (-9.3 kcal/mol). The rhodanine molecules based on this series have the potential to provide unique and effective clinical opportunities for treatment of diabetes.
Figure-1: Compound 4g binding mode inside the PPARγ active site; A) Compound 4g (orange sticks) is aligned on the 1WM0 co-crystallized ligand (blue sticks) along with the rosiglitazone bioactive conformation (red sticks); B) Compound 4g binding mode inside the PPARγ active site. The picture was generated by MOE. Hydrogen bonding is shown as orange dotted lines.
Recent Publications
- Shaikha S Al Neyadi, Alaa A Salem, Abdou Adem, Naheed Amir and Ibrahim M Abdou (2017) Synthesis, in vitro biological evaluation of new pyrimidines as Glucagon-Like peptide-1 Receptor Agonists. Bioorganic & Medicinal Chemistry Letters; 27(22): 5071-5075.
- Shaikha S Al Neyadi, Alaa A Salem, Mohammad A Ghattas, Noor Atatreh and Ibrahim M Abdou (2017) Antibacterial Activity and Mechanism of Action of the Benzazole Acrylonitrile-Based Compounds: in vitro, spectroscopy and docking studies. European Journal of Medicinal Chemistry; 136: 270-282.
Raed M. Al-Zoubi
Jordan University of Science and Technology, Jordan
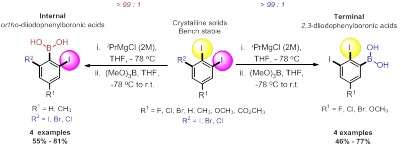
Title: Mild, Efficient and Regioselective Synthesis of Diiodophenyl boronic Acid Derivatives via Metal-Iodine Exchange of 5-Substituted-1,2,3-Triiodoarenes
Time : 12:20-12.45

Biography:
Raed M. Al-Zoubi has expertise in organic synthesis and boron methodology, he developed several boronic acid catalysts for green synthesis of several amide products providing water only as a byproduct in this process. Catalyst MIBA is commercialized nowadays by Sigma-Aldrich Company
Abstract:
A unique 2,6-diiodophenylboronic acid and 2,3-diiodophenylboronic acid derivatives have been synthesized via regioselective Metal-Iodine Exchange (MIE) of 5-substituted 1,2,3-triiodoarenes is reported. The regioselectivity of the reaction per se is remarkably controlled by the nature of C-5 substituent providing either the desired diiodophenylboronic acids in moderate to good yields and with high site-selectivity. The diiodophenylboronic acids were then examined for in-vitro antimicrobial activity against four strains of bacteria Micrococcus luteus (ATCC 9341), Bacillus Cereus (ATCC 11778), Escherichia coli (ATCC 25922) and Serratia marcescens (ATCC 27117) and one fungal strain Candida albicans using well diffusion assay and dilution method. It indicated that (5-fluoro-2,3-diiodophenyl)boronic acid (compound 16B) possess the most potent antibacterial and antifungal activity with MIC of 2.6 mg/mL for M. luteus and C. albicans. This report discloses a one-step protocol to access hitherto unknowns 2,6-diiodophenylboronic acid and 2,3-diiodophenylboronic acid derivatives that is scalable, good in scope, no chromatography is needed and indeed difficult to be prepared by other means.
Recent Publications (minimum 5)
[1] a) M. Bérubé, M. Dowlut and D. G. Hall, J. Org. Chem. 2008, 73, 6471-6479; b) W. Yang, X. Gao and B. Wang, Med. Res. Rev. 2003, 23, 346-368.
[2] a) R. M. Al-Zoubi and D. G. Hall, Org. Lett. 2010, 12, 2480-2483; b) R. M. Al-Zoubi, O. Marion and D. G. Hall, Angew. Chem. Int. Ed. 2008, 47, 2876-2879; c) N. Gernigon, R. M. Al-Zoubi and D. G. Hall, J. Org. Chem. 2012, 77, 8386-8400; d) T. Marcelli, Angew. Chem. Int. Ed. 2010, 49, 6840-6843.
[3] T. J. Zimmermann, M. Buerger, E. Tashiro, Y. Kondoh, N. E. Martinez, K. Goermer, S. Rosin-Steiner, T. Shimizu, S. Ozaki, K. Mikoshiba, N. Watanabe, D. Hall, I. R. Vetter, H. Osada, C. Hedberg and H. Waldmann, ChemBioChem 2013, 14, 115-122.
[4] T. Akama, K. Jarnagin, J. J. Plattner, S. R. Pulley, W. H. White, Y.-K. Zhang and Y. Zhou, Eli Lilly & Anacor Pharmaceuticals Inc. World patent WO2014149793A1, 2014, p. 160.
Shaista Shoukat
Forman Christian College, Pakistan
Title: Various medicinal compounds can act as ligands

Biography:
Shaista Shoukat is cureently working as a Chemistry lecturer at Forman Christian College, Pakistan.
Abstract:
Various medicinal compounds can act as ligands due to the presence of donor atoms such as nitrogen, oxygen and sulphur and form coordinate complexes with metal ions. As a result their structures are modified along with their medicinal properties and therapeutic effects. This is an effective way of improving their efficacy and toxicity. The present research encompasses the same concept by chelating anti-cancer drug methotrexate (MTX) with metal salts. Metal complexes of MTX were prepared with Zinc(II), Copper(II) and Chromium(III) which were characterized by elemental analysis, thermal analysis, powder XRD and other spectroscopic techniques. The spectral, thermogravimetric and elemental analysis data indicated that the synthesis of methotrexate complexes namely Zn-MTX, Cu-MTX and Cr-MTX exhibit yellow, light green and dark green colours, respectively. The proposed structures of Zn-MTX, Cu-MTX and Cr-MTX further suggested tetrahedral, square planar and octahedral geometries, respectively.
Biography:
Abstract:
Shaista Shoukat
Forman Christian College, Pakistan
Title: Various medicinal compounds can act as ligands
Time : 11:30-11:55

Biography:
Shaista Shoukat is cureently working as a Chemistry lecturer at Forman Christian College, Pakistan.
Abstract:
Various medicinal compounds can act as ligands due to the presence of donor atoms such as nitrogen, oxygen and sulphur and form coordinate complexes with metal ions. As a result their structures are modified along with their medicinal properties and therapeutic effects. This is an effective way of improving their efficacy and toxicity. The present research encompasses the same concept by chelating anti-cancer drug methotrexate (MTX) with metal salts. Metal complexes of MTX were prepared with Zinc(II), Copper(II) and Chromium(III) which were characterized by elemental analysis, thermal analysis, powder XRD and other spectroscopic techniques. The spectral, thermogravimetric and elemental analysis data indicated that the synthesis of methotrexate complexes namely Zn-MTX, Cu-MTX and Cr-MTX exhibit yellow, light green and dark green colours, respectively. The proposed structures of Zn-MTX, Cu-MTX and Cr-MTX further suggested tetrahedral, square planar and octahedral geometries, respectively.
- Drug Formulation/ Pharmaceutical formulation | Clinical Trials | Pharmacology and Toxicology | Drug Discovery Using Nanotechnology | Anti-Cancer Drug Discovery