Theme: An Innovative approach to the era of Drug Discovery and Toxicology Research
Drug Chemistry 2019
Track ​1: Drug Discovery and Development
Drug discovery is a process in pharmacy which involves the interaction of various disciplines leading to the invention of potent drug entities. The major part of drug design involves the identification of characteristic diagnostic biomarkers such as a protein responsible for the disease or disorder and then developing a drug molecule of therapeutic potency that targets it. The process involves various branches of pharmacology coalesce with biotechnology, bioinformatics, molecular biology, nanotechnology and biochemistry that ultimately leads to the production of molecules of therapeutic value. Despite the advancements in modern technologies and an understanding of the biological systems, the drug discovery process is still a lengthy and expensive task. There are only a few therapeutic drugs that pass the test and enter the market but today's accelerated studies using computational drug design techniques speed up the process of drug discovery.
- Diagnostic markers as therapeutic targets
- Approaches in target identification and validation
- In silico pharmacology
- Drug design and molecular modeling
- Molecular docking studies
Track 2: Drug Chemistry and Toxicology
Drug chemistry is a branch of pharmacy that overlaps with the disciplines of chemistry that involves drug design, chemical synthesis, drug formulation, drug testing, and development. Compounds used as medicines are usually organic or organometallic compounds. Today’s pharmaceutical industry evolved largely from the chemical industries. Modern advancements in biological sciences related to the structure and function of DNA as well as precise methods for manipulating DNA and making proteins has led to a more balanced partnership for drug discovery between chemistry and biology. The field of drug chemistry focuses on four key points to arrive at a compound of pharmaceutical importance, they are drug synthesis, interpretation of the chosen target to a defined pharmacodynamics and pharmacokinetics mechanism of drug action, drug screening to analyze blood, urine, hair, saliva or tissue samples to detect the presence residual chemicals and contaminants left behind in the body as a result of drug use, toxicity assessments and adverse drug reaction studies.
The drug toxicology studies play a very important role in the drug development process that evaluates the safety of potential drug candidates. Toxicity assessments are done using relevant animal models and validated procedures. Once a new drug entity is synthesized it is subjected to various in vitro and in vivo toxicity tests. The ultimate aim of it is to translate the animal model responses into predictable toxic responses in humans.
- Drug synthesis and pharmaceutical formulation
- Pharmacological interactions
- Mechanism of drug action
- Drug screening and toxicity assessments
- Adverse Drug Reactions
Track 3: Clinical Trails and Dosage Studies
A clinical trial is an examination program led with patients to evaluate a new treatment or medication. The motivation behind clinical preliminaries is to discover better strategies for treating, screening and diagnosing distinctive ailments. Clinical trials are responsible for the wide application of the latest scientific and technological advances to patient care. Each clinical trial has criteria describing the participants. Children, adults, healthy volunteers, patients and people of a diverse group of ethnic and racial foundations are urged to participate in clinical trials. Clinical preliminaries are partitioned into 4 stages, called clinical trial phases. The first two phase trials assess the drug for its lethality or the side effects it causes and later two-phase trials aim to test a new drug for its efficiency than the existing medications. Each new phase of a clinical preliminary expands data from past stages. A dose-ranging study is a part of phase II clinical trial where different doses of a drug molecule are tested to establish which dose is effective with least or no lethality. The dose-response relationships in clinical trials are necessary for determining a safe quantity of drugs. Thus the vast data accumulated by clinical trials form the basis for public policy and drug approval process. Once the drug enters the market it is subjected to post-market drug safety surveillance to assess further problems.
- Preclinical research
- Phases of clinical trial
- Drug approval process
- Pharmacokinetic and pharmacodynamics dosage studies
- Post-market drug safety monitoring
Track 4: Novel Drug Delivery Systems
Drug delivery is a process that includes approaches and techniques for transporting a pharmaceutical compound in the body is ordered to safely achieve its desired therapeutic effect. In order to minimize drug degradation, prevention of harmful side effects, increasing drug bioavailability and the amount of the drug accumulated in the precise zone of requirement various drug delivery and targeting systems are currently under development. Well-designed drug carriers that carry drug molecules are being innovated and utilized for site-specific drug delivery. Some of them include liposomes, micelles, dendrimers, liquid crystals, nanocapsules, and nanospheres. The mode of drug delivery is responsible for the drug’s success or failure as the choice of a drug is often influenced by the way the medicine is administered. The pulsatile release is a newly developed method of drug delivery as it mimics the glands that produce hormones in a controlled way. It is achieved by using drug-carrying polymers that respond to specific stimuli hence this system is called the stimuli-responsive system.
- Advanced pharmaceutical carriers
- Prodrugs
- Transdermal drug delivery
- Site-specific drug delivery
- Stimuli-responsive system
Track 5: Pharmacogenomics and Precision Medicine
The pharmacogenomics comprises of genomic information being used to study an individual’s responses to drugs based on their genetic profile. When a gene variant is responsible for a particular drug response in a patient then based on genetics it is possible to derive clinical decisions by adjusting the dosage or choosing an alternative drug. Researchers study gene variants affecting an individual's drug response in a similar process as of assessing variants associated with diseases and disorders. Recent approaches include multigene analysis or whole-genome Single Nucleotide Polymorphism (SNP) profiles which are used in designing and developing drugs. By the application of human genomic data a new technique called precision medicine is being established, which is based on using an individual's genetic data to make a best therapeutic choice by facilitating predictions about whether a particular person will be beneficial by a particular medicine or suffer by serious side effects. Drugs are tested on a large population of people and the average response is reported. Personalized medicine acknowledges that no two patients are similar with respect to reaction towards medication.
- Predictive prescribing
- Toxgnostics
- Clinomics
- Molecular medicines
- Drug safety
Track 6: Nuclear Pharmacy
Radioactive drugs or radiopharmaceuticals are drugs containing a radioactive component which are administered to humans for the purpose of diagnosis or treatment of a disease. These radioactive drugs are available in commercial nuclear pharmacies or can be synthesized in hospital-based nuclear pharmacies. In nuclear medicine industries, devices such as cyclotron and radionuclide generators are used to manufacture nuclear drugs with reduced risk condition. Radiopharmaceuticals play a vital role in curing diseases such as cancer, thyroid conditions, and polycythemia vera. Extensive research is going to make nuclear medicines safer by limiting the health risks of radiation sickness and inappropriate dosing.
- Use of radioisotopes in medicine
- Radiopharmaceuticals- design and manufacture
- Radionuclide therapy
- Radioimmunotherapy
- Radioactive iodine therapy
Track 7: Neurology and Behavioral Pharmacology
Neuropharmacology is the investigation of how medication influence cellular process in the nervous system and the neural mechanisms through which they impact on behavior. It is divided into two parts behavioral neuropharmacology and molecular neuropharmacology, the former focuses on the study of how drugs affect human behavior which includes the study of how drug dependence and drug addiction affects the human brain and later focuses on the study of neurons and their neurochemical interactions to develop drugs having beneficial effects on neurological health. Antidepressants, antianxiety, anticonvulsant and antipsychotic drugs are the most widely prescribed neuroactive medications.
- Neuroactive drugs
- Molecular Neuropharmacology
- Clinical trials in psychopharmacology
- Drug addiction
- Placebo medication
Track 8: Traditional Medicines and Herbal Therapy
Traditional medicine or ethnomedicine refers to the knowledge, skills, and practices based on the speculations, convictions, and experiences indigenous to different cultures used in the maintenance of health and to prevent, diagnose, improve or treatment of physical and mental illness. Herbal treatments are the most popular form of traditional medicine and about 70 to 80 percent of the regions have been using it across the world as a form of primary health care. The traditional medicinal knowledge is thought to be within everyone’s reach and does not require training to practice it. In the present day context contribution made by traditional medicine to modern medicine is enormous. Many well-established drugs such as leptospermone came from phytomedicine research and identification of bioactive molecules of the therapeutic significance of plants used traditionally by tribals and villagers.
- Bioprospecting
- Bioactive molecules
- Home remedies
- Nutraceuticals
- Folk medicines
Track 9: Prebiotics and Probiotics
Probiotics are live microorganisms or microbial mixtures administered to improve the patient's microbial balance thus curing the disease. On the other hand, prebiotics is certain substances which on administration stimulate the growth of beneficial bacteria which eventually leads to curing of the disease. These are usually non-digestible carbohydrates found in fibrous foods. The mixture of probiotics and prebiotics is called synbiotics. Such medicines have wide applications in the treatment of various gastrointestinal disorders, irritable bowel syndrome, urinary tract infections, etc. The effectiveness and less health risk make probiotic drugs a promising drug for the future by replacing conventional medicines for certain diseases.
- Probiotic microbiota and synbiotics
- Enteric microflora and its therapeutic manipulation
- Prebiotic sources and its production strategy
- Health risks and benefits of probiotics
- Future aspects of probiotics
Track 10: Nanotechnology in Drug discovery
In recent years nanotechnology has found its significant interest in a wide range of applications in the pharmaceutical industry. Nanomaterials have been used in in-vivo preclinical and clinical trials. The choice of a particular technique depends on a variety of factors such as administration route, dose, drug physicochemical properties, drug target, and target cells or tissues. Many diverse nano-sized structures have been investigated for drug formulation and delivery. The nano-sized crystalline drug, drug-polymer and drug-antibody conjugates, dendrimers, liposomes, lipid emulsions, and solid drug-polymer nanoparticle dispersions are some of the drug carriers containing nanomaterial.
- Therapeutic properties of nanomaterials
- Nanoparticles as drug vehicles
- Role of nanotechnology in drug design and manufacture
- Biocompatibility of nanoparticles
- Regenerative Therapeutics
Track 11: Discoveries in Cancer Chemotherapy
In the current scenario, the most commonly used types of cancer treatment are chemotherapy, radiotherapy, tumor surgery, and hormonal therapy. Small molecule inhibitors are being extensively studied for precise target treatment. Their extremely small size allows them to diffuse through the plasma membrane and interact with the cytoplasmic domain of cell-surface receptors and intracellular signaling molecules that eventually blocks cancer cell proliferation. The application of genomic data in the field of oncology has given an insight into genetic modifications and epigenetic modifications such as chromatin-modification and DNA Methylation. Use of bioinformatics and proteomics has further speeded up the drug discovery process in cancer.
- Small molecule inhibitors as cancer therapeutics
- Tumor angiogenesis inhibition
- Chemotherapy-induced autophagy
- Oncogenomics in drug design
- Oncoproteomics based drug development
Track 12: Drug Resistance
Drug resistance or antimicrobial resistance is a phenomenon where microorganisms such as bacteria, fungi, viruses, and parasites change and develop resistance towards treatment by antimicrobial drugs rendering it ineffective hence infection persists in the body increasing the risk. Drug resistance occurs naturally over time by frequent misuse or overuse of antimicrobial drugs via genetic changes. Advancements in molecular and biochemical techniques have given an insight into antibiotic resistance genes and mechanisms such as mutation and gene transfer through which bacteria develop resistance. Antibiotic resistance is a major menace in modern-day therapeutics. In order to reduce the drug-induced resistance in bacteria, many antibiotic replacement therapies are put to use, such as phage therapy, use of bacteriocin, predatory bacteria and by competitive exclusion.
- Multidrug resistance proteins
- Antimicrobial resistance
- Genetics of drug resistance
- Biochemical aspects of drug resistance
- Phage therapy as an alternative to antibiotics
Track 13: Drug Tolerance
Drug tolerance is a phenomenon of adaptation for frequent exposure to a drug that results in a diminution of one or more of the drug's effects over time. The drug tolerance is classified into 3 types, which are acute tolerance (short-term, tolerance caused by repeated exposure to a drug for short period of time), chronic tolerance (long-term tolerance that develops when an individual’s body adapts to constant exposure to a drug over weeks or months) and behavioral tolerance (Due to overuse of certain psychoactive drugs). The drug tolerance development is reversible and it depends upon both physiology and psychology of individuals.
- Tachyphylaxis
- Pharmacodynamic and metabolic tolerance
- Cross-tolerance
- Rebound phenomenon and drug desensitization
- Drug dependence
Track 14: Clinical Toxicology and Xenobiotics
Clinical Toxicology involves the study of the toxicity of substances that are related to therapeutics or healthcare. It contains the interaction of various disciplines such as pharmacology, environmental toxicology, biochemistry, computational toxicology, etc. The particular field focuses on treatment techniques of patients who have been poisoned with a drug or other substance either accidentally or intentionally. The application of this is also used in forensic sciences. Toxicology studies mainly rely on xenobiotics which are substances foreign to the particular biological system such as drugs, environmental toxins, food additives, etc. Hence identification, mechanism and studying its metabolism give information on various ways to treat fatality.
- Ecotoxicology with a health perspective
- Forensic toxicology
- Target organ toxicity and cure
- Xenobiotic metabolisms
- Xenotransplantation- A futuristic view
Track 15: Biotechnology Processes in Pharmaceutics
Pharmaceutical biotechnology is the reconciliation of pharmacy with various scientific orders that include biochemistry, microbiology, genetics, organic chemistry, and chemical engineering. It utilizes living systems or products from the biological system to make or alter valuable biological substances with therapeutic value. Modern application of biotechnology in pharmacy involves hereditary building, protoplast fusion; monoclonal antibody systems and powerful new devices intended to create effective bioprocess technology and products for the growth of pharmaceutical industries.
- Genetic engineering to aid drug discovery
- Monoclonal antibodies
- Molecular biotechnology
- Bioprocessing of drugs
- Applications of systems biotechnology in medicine
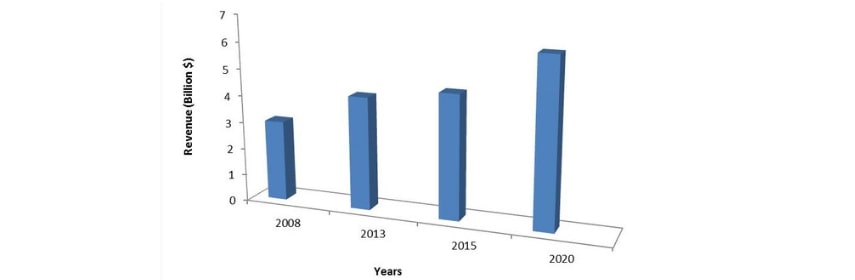
The pharmaceutical market in the United Arab Emirates (UAE) witnessed a growth of $3 billion in 2015 and is estimated to become $6 billion in 2020. The substantial growth is driven by increased expenditure on healthcare, mandatory health insurance, and growing medical tourism.
As per the Global Data’s latest research report, the UAE is increasingly gaining popularity as a medical tourism destination due to its low costs of medicines, medical staff with good English fluency and virtually non-existent queues for treatment. The Dubai Health Authority is on a mission to develop the medical tourism sector and strengthen it. It aims to attract about 1.3 million medical tourists annually by 2021. The revenue on medical tourism is expected to grow about 13% annually for the next five years. Another major initiative taken by UAE government is allowing private sector involvement and investment in the development of healthcare with this decision the UAE government's share of total health expenditure fell over from 71.3% in 2015 and estimated to become 60.7% by 2025.
In a country like UAE were urge for healthcare is more, conferences based on drug discovery process play a very important role in bringing the latest skills and techniques making the process of design, screening, manufacture and analysis cost effective. It would bridge the gap between the present day pharmacy and the public which would lead to greater understanding of the healthcare and give a scope for further development.
The Global Market Scenario
The world pharmaceutical market is developing consistently every year. As per current market analysis report by the Quintiles IMS Institute, costs for medications will add up to 1.5 trillion U.S. dollars comprehensively in 2021. These equivalents a normal yearly development rate of somewhere in the range of 4% and 7%. The USA pharmaceutical market will witness the largest growth. The increase in urbanization and growth of middle-class peoples has made the drugs available to many people at affordable prices. This development leads to a higher demand for medication thus increasing the revenue of pharmaceutical companies.
Why Abu Dhabi?
Abu Dhabi has a well-established healthcare system with public and private healthcare facilities. There are many pharmacies, hospitals, and clinics which provide services round the clock. There are numerous primary healthcare centers which collaborate with public hospitals to offer specialized care and transfer patients to different units whenever required. Laws governing the marketing of drugs are very strict and medications such as sleeping pills and anti-depressants are prohibited from being sold without a proper prescription. Apart from primary health care centers, there are various other types of medical facilities such as Ambulatory care and Family medicine clinics and many of these facilities also train medical students.
UAE Department of Health has launched “Abu Dhabi Healthcare Strategic Plan” that urges the development of health care, pharmacy and patient safety system. It also encourages private sector involvement in healthcare development. It has recently launched a major drive to attract more medical tourists to the city by highlighting areas of medical excellence offered that cannot be found anywhere in the region and aims to transform Abu Dhabi into a medical tourism hub.
Related Societies & Associations
UAE
Emirates Medical Associations, Medicina Group of Pharmacies, Emirates Health Informatic Society, Emirates Pharmacy Society, Pharmacist Society, International Pharmaceutical Federation (FIP), International Pharmaceutical Students' Federation, Gulf Drug, Society of Critical Care Medicine, WFSA (World Federation of Societies of Anesthesiologists), Middle East Society of Sexual Medicine, Society for Simulation in Healthcare, Emirates Society of Emergency Medicine, Healthplus Network of Speciality Centers, Life Style Medicine Global Alliance, MECOMED, Emirates Physiotherapy Society, Radiology Society of Emirates, Healthpoint, UAE Genetic Diseases Associations, Emirates Society of Rheumatology, Emirates Urology Society, Emirates Osteoporosis Society, Pan Arab Interventional Radiology Society, AACC (American Association For Clinical Chemistry), Emirates Nursing Association, Emirates Plastic Surgery Society, Emirates Dermatology Society, Dental Society, Emirates Cardiac Society, Emirates Neurology Society, Emirates Clinical Nutrition Society, Emirates Society Of Mental Health, Emirates Society of Haematology, UAE Nursing and Mid-wifery Council.
Middle-East
Bahrein Medical Society, Bahrain Pharmacists Society, Saudi Nursing Society, International Iraqii Medical Association, EUROTOX, SETAC Arabian Gulf Branch, Himss Middle East, Saudi Critical Care Society, WHO Collaborating Centre for Education Development for Health Professions, Oman Dermatology Society.
Related Conferences
- 6th International Conferences on Trends in Health and Medicine, December 28-29, 2018, Paris, France.
- 2nd Allergy & Respiratory Medicine Conferences, January 18, 2019, Abu Dhabi, UAE.
- International Conferences on Biomarkers and Clinical Research, January 21-22, 2019, Dubai, UAE.
- International Conferences on Medicine, Nursing and Healthcare, January 23-24, 2019, Dubai, UAE.
- International Conferences on Pediatric Nursing and Healthcare, January 23-24, 2019, Dubai, UAE.
- 11th International Research Conferences on Science, Health and Medicine 2019, January 24-25, 2019, Dubai, UAE.
- 27th International Conferences on Nanomedicine and Nanomaterials, January 25-26, 2019, Dubai, UAE.
- International Conferences on Molecular Markers and Cancer Therapeutics, January 25-26, 2019, Dubai, UAE.
- 5th Annual Arab Paediatric Medical Congress, February 14-16, 2019, Dubai, UAE.
- 2nd Middle East Pharmacy and Pharmaceutical conferences, February 14-15, 2019, Dubai, UAE.
- 2nd International Conferences on Integrative Biology & Medicine, February 18 – 20, 2019, Dubai, UAE.
- 11th World Congress on Complementary and Alternative Medicine, February 25-26, 2019, Dubai, UAE.
- 2019 International Conferences on Research in Life-Sciences & Healthcare, February 27-28, 2019, Dubai, UAE.
- Global Congress on Oncology and Cancer Research, March 04-05, 2019, Dubai, UAE.
- 3rd Annual Dubai International Musculoskeletal Medicine Congress, March 06 – 07, 2019, Dubai, UAE.
- 12th International Conferences on Pharmacoepidemiology and Clinical Research, March 18-19, 2019, Dubai, UAE.
- 28th World Neonatal, Pediatric and Family Medicine Conferences, March 21-22, 2019, Dubai, UAE.
- 12th World Conferences on Human Genomics and Genomic Medicine, April 8-9, 2019, Abu Dhabi, UAE.
- 3rd International Conferences on Molecular Medicine and Diagnostics, April 11-12, 2019, Abu Dhabi, UAE.
- Middle East Pharma Cold Chain Congress, April 17-19, 2019, Dubai, UAE.
- 2nd Middle East Laboratory and Diagnostics Congress, April 18-20, 2019, Dubai, UAE.
- 3rd World Congress on Public Health and Health Care Management, April 19-20, 2019, Dubai, UAE.
- 6th World Summit on Cancer research and Therapy conferences, April 19-20, 2019, Dubai, UAE.
- International Summit on Biotechnology & Healthcare, April 22-24, 2019, Dubai, UAE.
- 6th GCC Healthcare Innovation Congress, April 30 -May 1, 2019, Dubai, UAE.
- 6th International Conferences on Rare Diseases & Orphan Drug, May 13-14, 2019, Dubai, UAE.
- 2nd International Conferences on Molecular Biology and Medicine, May 16-17, 2019, Dubai, UAE.
- 6th World Congress on Advanced Nursing and Healthcare, June 13-15, 2019, Brussels, Belgium.
- 22nd World Congress on Nursing, Pharmacology and Healthcare, July 10-11, 2019, Dubai, UAE
- Diagnostic Microbiology and Infectious Diseases Summit, September 09-10, 2019, Dubai, UAE.
- 2nd World Congress on Traditional and Complementary Medicine, September 16-17, 2019, Dubai, UAE
- Addiction, Alcoholism, Drug-Substance Abuse, Psychiatry and Recovery Summit, October 07-08, 2019, Abu Dhabi, UAE.
- 4th Advanced Medicine Congress, November 16-17, 2018, Abu Dhabi, UAE
- WONCA World conference 2020, November 26 – 29, 2020, Abu Dhabi, UAE.
Drug Discovery Congress 2018
We gratefully thank all our wonderful Speakers, Conference Attendees, Students, Media Partners, and Associations for making Drug Discovery Congress 2018 Conference the best ever!
The 5thAnnual Congress on Chemistry in Drug Discovery & Designing, hosted by the Conference series LLC Ltd was held during April 16-17, 2018 at Dubai, UAE based on the theme “Novel Strategies and Technologies for Drug Discovery and Medicinal Chemistry". Benevolent response and active participation was received from the Organizing Committee Members along with Scientists, Researchers, Students and leaders from various fields of Pharmaceutical sciences and Drug Discovery, who made this event a grand success.
Conference Series LLC Ltd expresses its gratitude to the conference Moderator, namely Dr. Heyam Saad Ali for taking up the responsibility to coordinate during the sessions. We are indebted to your support.
The conference was initiated with the Honorable presence of the Keynote forum. The list includes:
- Dale W Laird, The University of Western Ontario, Canada
- Mustafa Guzel, Istanbul Medipol University, Turkey
- Mahmoud Salama Ahmed, British University in Egypt, Egypt
- Anita Yadav, University of Mumbai, India
- Mohammed Al- Aljaley, Mosul University, Iraq
The meeting reflected various sessions, in which discussions were held on the following major scientific tracks:
· Drug Discovery Using Nanotechnology
· Pharmaceutical Research & Development ( R&D )
· Clinical Trials and Regulatory Affairs
· Anti-Cancer Drug Discovery
· Natural Products Drug Discovery
· Drug Design and Drug Development
· Pharmacology and Toxicology
· Perspective in Drug Discovery
· Hot Topics in Drug Targets
· Drug Design and Drug Development
· Process Chemistry and Drug Manufacturing
Conference series LLC Ltd thank all our expert presenters from all around the world which includes various outside experts, University representatives and other eminent researchers who supported the conference by facilitating the discussion forums.
With the grand success of Drug Discovery Congress 2018, Conference Series LLC Ltd take the immense pleasure to announce the “6th Annual Congress on Drug discovery & Toxicology ” to be held during July 15-16, 2019 at Abu Dhabi, UAE
For More details visit: http://drugchemistry.pharmaceuticalconferences.com/
Conference Highlights
- Drug Discovery and Development
- Drug Chemistry and Toxicology
- Clinical Trails and Dosage Studies
- Novel Drug Delivery Systems
- Pharmacogenomics and Precision Medicine
- Nuclear Pharmacy
- Neurology and Behavioral Pharmacology
- Traditional Medicines and Herbal Therapy
- Prebiotics and Probiotics
- Nanotechnology in Drug discovery
- Discoveries in Cancer Chemotherapy
- Drug Resistance
- Drug Tolerance
- Clinical Toxicology and Xenobiotics
- Biotechnology Processes in Pharmaceutics
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | July 15-16, 2019 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | |||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
Abstracts will be provided with Digital Object Identifier by


